Transform clinical trials and medical device rollouts with secure, validated translation management that meets GxP standards and international regulatory requirements. Our platform combines intelligent automation with expert oversight for pharmaceutical documentation, clinical studies, and device localisation.
XTM Cloud gives your team a single, secure environment to manage translations across trials and device launches. Whether it’s regulatory submissions, patient consent forms, or multilingual instructions, every file is tracked, validated, and ready for audit. The result is fewer delays, faster approvals, and confidence that compliance standards are met in every market.


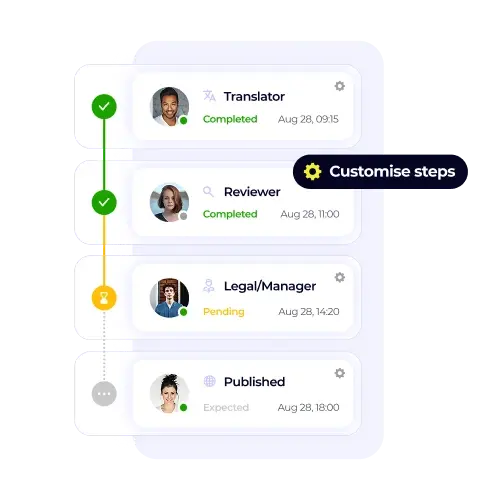
/USPs%20-%20Multi-format%20file%20support.webp)







.jpg)







.png?width=206&height=206&name=frame%20(19).png)






-2.png?width=280&height=280&name=Untitled%20design%20(4)-2.png)